Corneal nerves are important for cornea health, and for protecting the eye from outside elements. They are
mostly nociceptive, coding discomfort and pain in response to mechanical stimulation, temperature and/or
chemical stimulation. Disease, infection and ocular surgery all damage corneal nerves, with long-term
consequences in terms of pain, dry eye, recurrent erosions, opacity and even blindness. Yet, there are no
effective clinical therapies for nerve dysfunction once fibrosis has occurred. Our prior work allowed us to better
understand factors that control myofibroblast differentiation in the large wounds generated in human and cat
corneas. This work also led to the observation of a nefarious interaction between wound myofibroblasts and
regenerating corneal nerves. Pre-treating the wound area with anti-fibrotic agents can mitigate this problem,
but in most cases of accidental injury or infection, patients present after myofibroblast differentiation/fibrosis
have occurred. The issue then is no longer preventing fibrosis, but rather overcoming the contact inhibition
between nerves and persistent myofibroblasts. We recently found that the PPARg ligand Troglitazone
stimulates neurite outgrowth and causes myofibroblasts de-differentiation in vitro and in vivo. Importantly,
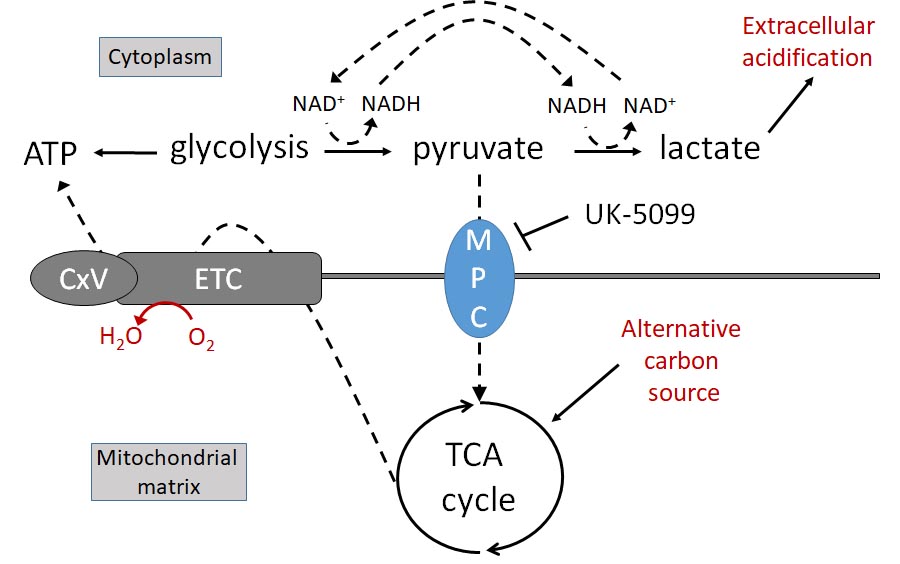
these effects can be mimicked in vitro by a compound that spares PPARg and targets the mitochondrial
pyruvate carrier (MPC). Together with recent literature, our pilot data form a strong premise for critically testing
the hypothesis that inhibiting the MPC may facilitate metabolic remodeling in both regenerating nerves and
activated myofibroblasts to overcome the blocked reinnervation inherent in later stages of post-injury corneal
fibrosis. To test this hypothesis, we will: Aim 1, identify metabolic changes through which inhibiting
mitochondrial pyruvate transport promotes neurite outgrowth of peripheral sensory neurons in a simulated
wound environment; Aim 2, assess if similar or different mechanisms (as in Aim 1) underlie de-differentiation
of corneal myofibroblasts; and Aim 3, contrast the relative efficacy of different mitochondrial modulators for
overcoming myofibroblasts’ inhibition on corneal nerve regeneration in vivo. Fundamentally, the proposed
research will ascertain if MPC activity is key to overcoming fibrosis and/or its associated inhibition of neurite
outgrowth. We will confirm target specificity through genome manipulation in both corneal fibroblasts and
peripheral sensory neurons, determine how targeting impacts mitochondrial function, and identify signals
downstream of metabolic rewiring that are necessary to mediate these effects. Since the MPC has never been
studied in the context of peripheral wounding, our results could unveil new, broadly-relevant aspects of its
function. Ultimately, the proposed research aims to unravel novel molecular mechanisms that may play a
substantial, hitherto unexplored role in large, established, fibrotic wounds. Defining these mechanisms is
necessary if we are to develop new therapies for the treatment of mature corneal wounds with an eye to
promoting optimal nerve regeneration and ensuring long-term corneal health and clarity.
Corneal Wound Healing and Nerve Regeneration

There are no comments
Add yours